Last week February 18 Pfizer and BioNTech announced a global Phase 23 trial of their COVID-19 vaccine in 4000 healthy pregnant women. Data on vaccine safety and immunogenicity in these populations are therefore limited.

The Coronavirus Vaccine Presents A Dilemma For Pregnant Women The New Yorker
In addition to vaccination protecting women against Covid-19 and its complications during pregnancy emerging evidence has shown transplacental transfer of severe acute respiratory syndrome.

Covid vaccine pregnancy trial. The first COVID-19 vaccine study in the UK recruiting pregnant women has been launched across several National Institute for Health Research NIHR sites including University Hospital Southampton. The phase 23 study aims to further understand the safety tolerability and immunogenicity of the Pfizer-BioNTech COVID-19 vaccine in healthy. To evaluate the immunogenicity of COVID-19 messenger RNA mRNA vaccines in pregnant and lactating women including against emerging SARS.
For each exposed pregnancy 2 unexposed pregnancies enrolled in the IRCEP will be matched by country and gestational age at enrollment - 1 week. A new observational study has begun to evaluate the immune responses generated by COVID-19 vaccines administered to pregnant or postpartum people. Led by researchers at Maccabi Healthcare Services in Tel Aviv the retrospective observational study was published yesterday in JAMA.
The placebo-controlled observer-blinded study will track the safety tolerability and immunogenicity of two doses of vaccine 21 days apart compared to placebo for 7 to 10 months. Vaccines that use the same viral vector have been given to pregnant people in all trimesters of pregnancy including in a large-scale Ebola vaccination trial. Called developmental and reproductive toxicity DART studies research with pregnant animals can provide reassurance about moving forward with vaccine research in pregnant people.
Lack of trials in pregnant people driving Covid-19 vaccine hesitancy. At least 200 pregnancies exposed to each branded COVID-19 vaccine during the first trimester and 300 exposed thereafter during pregnancy are projected. Two doses of the PfizerBioNTech COVID-19 vaccine were safe and 78 effective in preventing infection in pregnant women in a real-world study in Israel.
No adverse pregnancy-related outcomes including adverse outcomes affecting the baby were associated with vaccination in these trials. Participant has received any other COVID-19 vaccines at any point from 28 days prior to LMP throughout pregnancy. Pfizers trial will include 4000 pregnant women.
Researchers will measure the development and durability of antibodies against SARS-CoV-2 the virus that causes COVID-19 in people vaccinated during pregnancy or the first two postpartum months. Vaccinations have been postponed or cancelled in at least 40 states due to weather. The data so far suggest that it is and given the increased risks associated with COVID-19 in pregnancy many pregnant people have decided to accept the vaccine.
Women currently participating in another investigational device or drug study currently taking an investigational medicinal product or having taken an investigational product within 28 days prior to LMP or during pregnancy. By Kezia Parkins 22 Jul 2021 Last Updated August 11th 2021 1520 Studies testing the safety of Covid jabs in pregnant people have only just begun leaving some hesitant to undergo vaccination. The Joint Committee on Vaccination and Immunisation JCVI has advised that pregnant women should be offered COVID-19 vaccines at the same time as people of the same age or risk group.
The expectation was that they would work just as well to protect pregnant women. Some COVID-19 vaccine makers have since planned to enroll pregnant people in their clinical trials. The drugmaker aims to.
COVID vaccine trial for pregnant women Pfizer and BioNTech say the first US. It is notable that as of April 26 2021 more than 100000 pregnant women reported having received a Covid-19 vaccination and yet only a small fraction 47 have enrolled in the v. Studies Confirm COVID-19 mRNA Vaccines Safe Effective for Pregnant Women.
Participants have been given shots in a large-scale clinical trial to assess the safety and efficacy of their COVID-19. We have summarized several academic papers that investigate outcomes of COVID-19 infection and the vaccines against it among pregnant individuals to help journalists bolster their reporting with data. The initial advice from immunisation expert groups was therefore cautious and COVID-19 vaccines were not routinely recommended in pregnancy.
The pandemic has shone a light on the historic failing and exclusion of. Pfizer launches COVID-19 vaccine trial in pregnant women. No adverse pregnancy-related outcomes occurred in previous clinical trials that used the same vaccine platform as the JJJanssen COVID-19 vaccine.
The countrys largest clinical trial investigating the best gap between first and second COVID-19 vaccine doses for pregnant women is being launched in England today Tuesday 3 August. Pregnant women are at increased risk of morbidity and mortality from COVID-19 but have been excluded from the phase 3 COVID-19 vaccine trials. Pregnant people were not included in the first clinical trials for COVID-19 vaccines so at the time of initial guidance there was limited evidence confirming the safety of COVID-19 vaccines during pregnancy.
The exclusion of pregnant and lactating women from COVID-19 vaccine trials Text Box 1 reflects a historic pattern of protection by exclusion representing an instance in which the estimated effect of a therapy on mother and child will rely on anecdotal and delayed reports from healthcare settings rather than the monitored setting of a clinical trial2 This exclusion is not justified. Clinical trials have shown that COVID-19 vaccines are remarkably effective in protecting those age 12 and up against infection by the coronavirus SARS-CoV-2. Pfizer-BioNTech said Thursday that it is beginning clinical trials of its Covid-19 vaccine in pregnant women the first such trials to include expectant mothers in the US.
By monitoring the outcomes for these people and their babies we will soon be able to make evidence-based recommendations on whether the vaccines should be rolled out to pregnant. A clinical trial called Preg-CoV has been launched to help determine the best gap between doses for pregnant women as well as exploring in greater detail. Although there is not yet pregnancy-specific data about COVID vaccines from clinical trials the vaccines have been studied in pregnant laboratory animals.

Covid Vaccines Don T Damage Placenta May Be Safe In Pregnancy Study Lifestyle News The Indian Express

Questions About The Covid 19 Vaccine Multnomah County

Opinion Far Too Many Pregnant People Remain At High Risk Of Covid 19 It Didn T Have To Be This Way The Washington Post
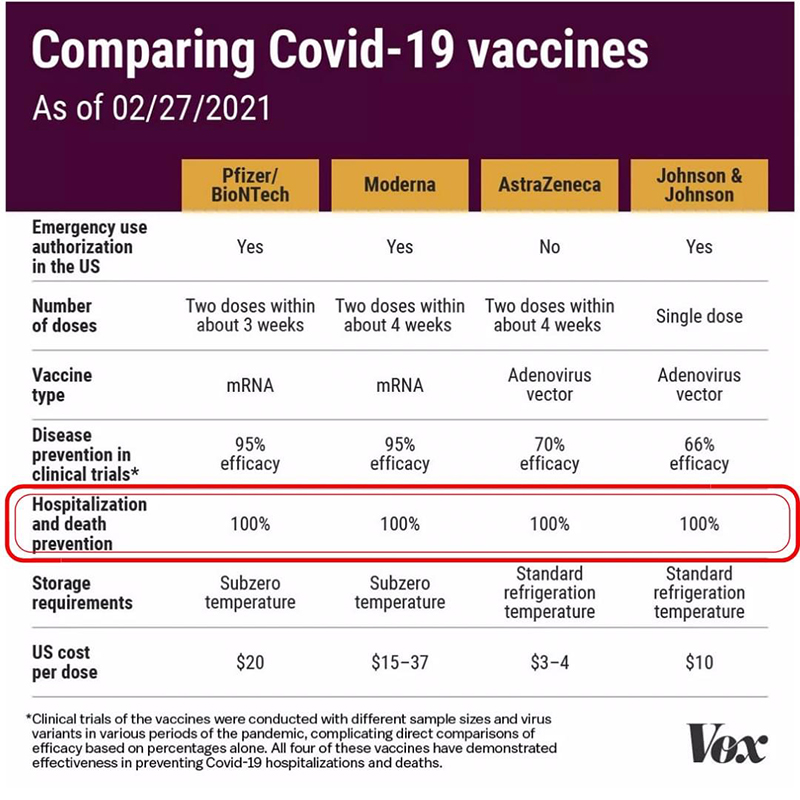
Covid 19 In Pregnancy Maternal Care Maternal Fetal Care High Risk Obstetrics Ur Medicine Obstetrics Gynecology University Of Rochester Medical Center

Pregnant People Haven T Been Part Of Vaccine Trials Should They Get The Vaccine Npr

Daily Dose Pregnant Or Trying To Get Pregnant Here S What You Should Know About The Covid 19 Vaccine

Should You Get The Covid 19 Vaccine While Pregnant Here S What Experts Say

Should Pregnant Women Be Vaccinated Against Covid 19
Should Pregnant Or Breastfeeding Women Take The Covid 19 Vaccine Covid Your Pregnancy Matters Ut Southwestern Medical Center
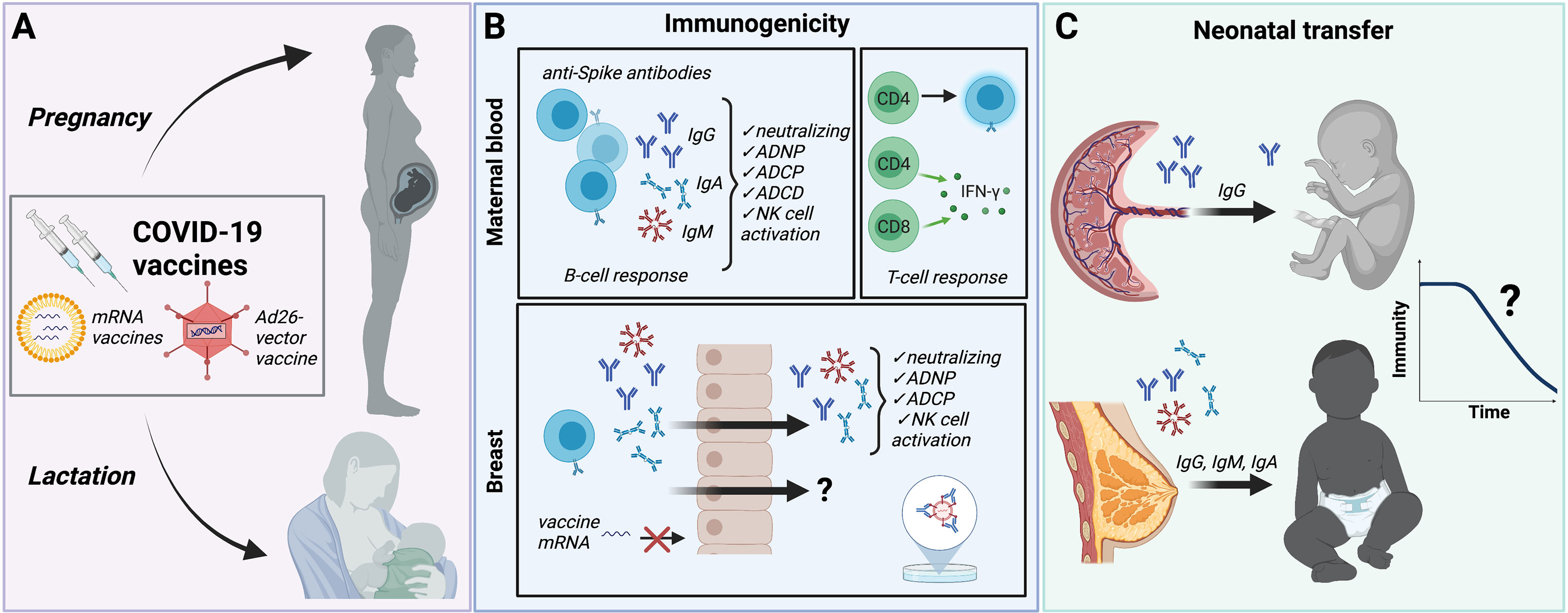
Frontiers Covid 19 Vaccination In Pregnancy And Lactation Current Research And Gaps In Understanding Cellular And Infection Microbiology
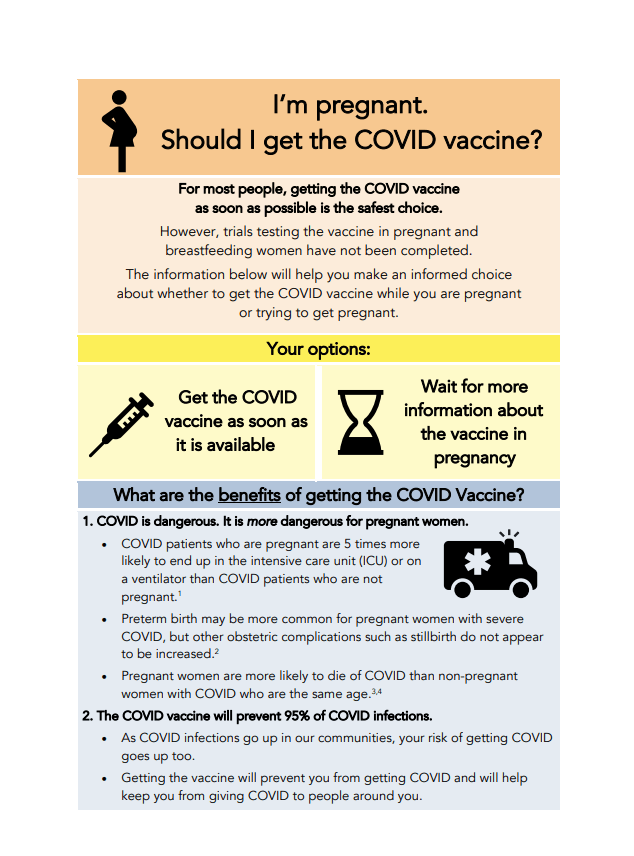
Covid 19 Vaccine Information For Pregnant Women Gender Covid 19
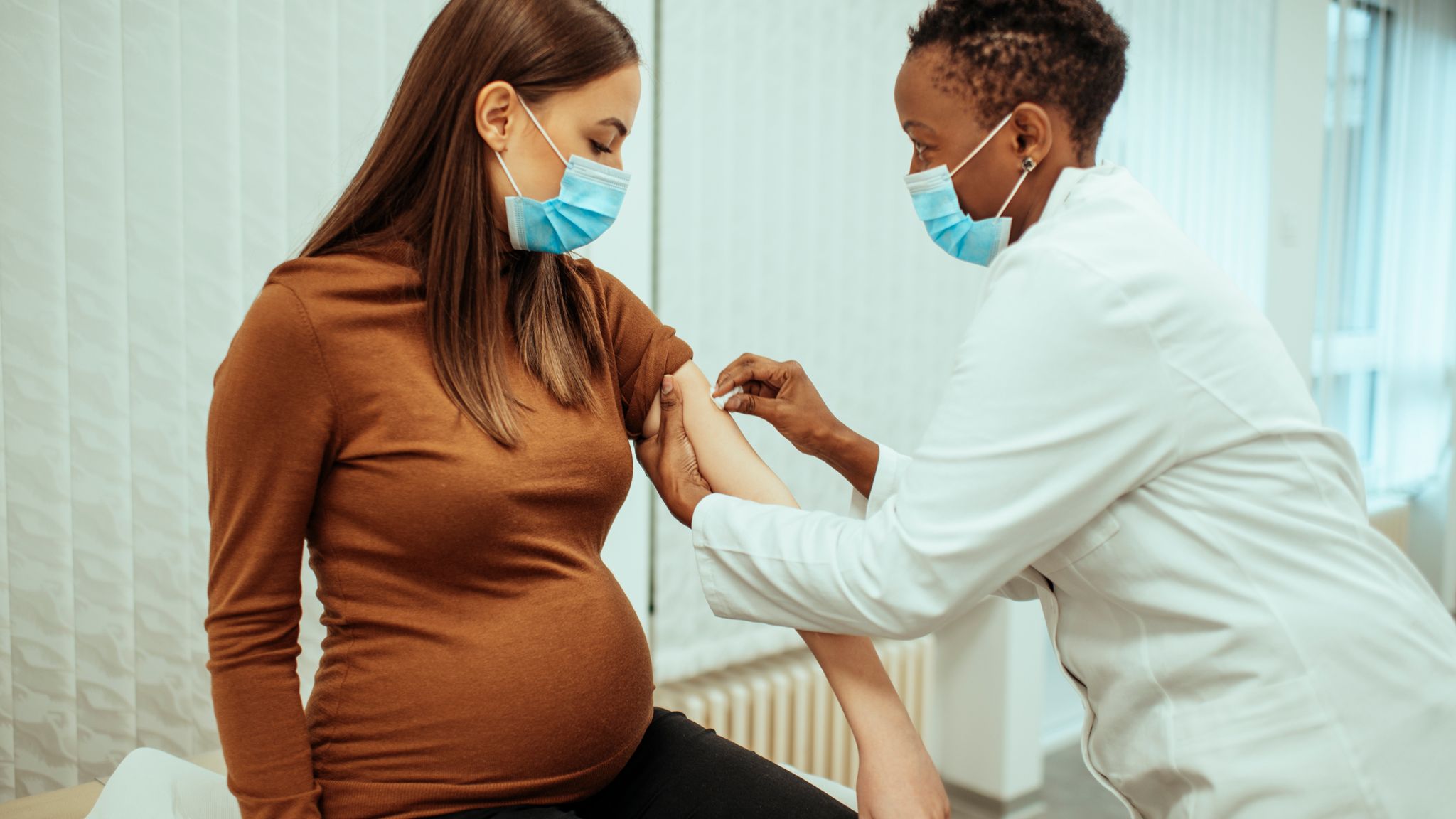
Covid 19 Trial To Find The Best Gap Between Vaccine Doses For Pregnant Women Uk News Sky News

Covid 19 Vaccine Study For Pregnant Women Launches The University Of Edinburgh

Pfizer To Study Covid 19 Vaccine On Pregnant Women

Will Covid 19 Vaccines Be Safe For Children And Pregnant Women
/GettyImages-1237987894-e8c1184324dc458eb541bcb7cd14d7e4.jpg)
Fda Pregnant Women Can Get A Covid 19 Vaccine

Pfizer And Biontech Begin Covid 19 Vaccine Trial In Pregnant Women

Pfizer Vaccine Pregnancy Trial University Of Southampton
Should Pregnant Women Be Vaccinated For Covid 19 Mayo Clinic
Covid Vaccine Pregnancy Trial. There are any Covid Vaccine Pregnancy Trial in here.
Popular Posts
Search Here
Arsip
-
▼
2021
(511)
-
▼
October
(52)
- Villahermosa
- Vecinos
- Vecinos Serie
- Tottenham
- Tottenham Vs. Manchester United
- Inter De Milan
- Inter De Milan Vs
- Inter De Milan Vs Napoli
- Inter De Milan Plantilla
- Coquimbo Unido Vs
- Coquimbo Unido Vs Junior
- Coquimbo Unido Vs Defensa Y Justicia
- Coquimbo Unido Sudamericana
- Club Brujas Manchester City
- Covid Vaccine Pregnancy
- Covid Vaccine Pregnancy Which Week
- Covid Vaccine Pregnancy Us
- Covid Vaccine Pregnancy Uk
- Covid Vaccine Pregnancy Trial
- Colon Hoy Televisado
- Colon Hoy Tabla De Posiciones
- Colon Hoy Resultado
- Colon Hoy Partido
- Colon Hoy Horario
- Gerd Ruge Kinder
- Freiburg Vs Rb Leipzig
- Freiburg Vs Rb Leipzig Results
- Freiburg Vs Rb Leipzig Prediction
- Freiburg Vs Rb Leipzig Head To Head
- Jodie Comer Help
- Jodie Comer Age
- Jo Cox Sister
- Ekleri Recepta
- Ordenado Minimo 2022
- Nuno Mendes
- Gabby Petito Update Boyfriend
- Gabby Petito Instagram
- Gabby Petito Freund
- Andy Murray Wife Engagement Ring
- Andy Murray Wife Age
- Andy Murray Wedding Ring
- Clasificatorias Sudamericanas
- Clasificatorias Qatar 2022
- Clasificatorias Qatar 2022 Chile
- Formel 1 2021 Punkte Tabelle
- Formel 1 2021 Ps4 Gameplay
- F1
- Diplomatisch
- Diplomat
- Diplomacy Is Not An Option
- UEFA Nations League
- Toma De Rehenes
-
▼
October
(52)
